- All
- Product Name
- Product Keyword
- Product Model
- Product Summary
- Product Description
- Multi Field Search
| 6 Resuts : | |
|---|---|
| 5 Resuts : | |
| 4 Resuts: | |
| 3 Results : | |
| Availability: | |
| Quantity: | |







ICA2114
BioTeke
A. Intended Use:
When it comes to Covid 19, Flu A, Flu B ,Respiratory Syncytial Virus (RSV) ,Adenovirus(ADV) and Mycoplasma Pneumoniae(MP) testing, Results from Bioteke SARS-Cov-2/Flu A /Flu B /RSV/ADV /MP antigen test kit allow physicians to make a rapid diagnosis with confidence, which is essential for reducing secondary testing, decreasing unnecessary antibiotic use, and guiding appropriate antiviral therapy.
B. Advantages:
• Patent design - Two-way chromatography ,only single sample well.
• Compact card better friendly to the environment.
• Multi-color display results,Easy-to-read results in 15 minutes.
• High Sensitivity, Specificity and Accuracy, reliable results.
• Long shelf life to adapt to seasonal fluctuations.
• Variety of collection and transport options.
• Convenient room temperature storage.

C.Result Analysis
• SARS-CoV-2: Specificity: 100% ; Sensitivity 96.08% ; Total Coincidence Rate : 99.47%
• Influenza A : Specificity: 100% ; Sensitivity 98.4% ; Total Coincidence Rate : 99.82%
• Influenza B : Specificity: 100% ; Sensitivity 96.8% ; Total Coincidence Rate : 99.65%
• RSV : Specificity : 100% ; Sensitivity 97.98% ; Total Coincidence Rate : 99.82%
• Adenovirus : Specificity : 100% ;Sensitivity 95.83% ; Total Coincidence Rate : 99.56%
• M.Pneumoniae : Specificity: 100% ; Sensitivity 97.98% ; Total Coincidence Rate : 99.82%
D. Four Options:
a. SARS-CoV-2/Flu A/Flu B/RSV/ADV/MP Antigen Rapid Test Kit (6 Results of Diseases)
This kit can be detected six items: SARS-CoV-2 , Influenza A virus , Influenza B virus , Respiratory syncytial virus , Adenovirus antigen and Mycoplasma Pneumoniae.

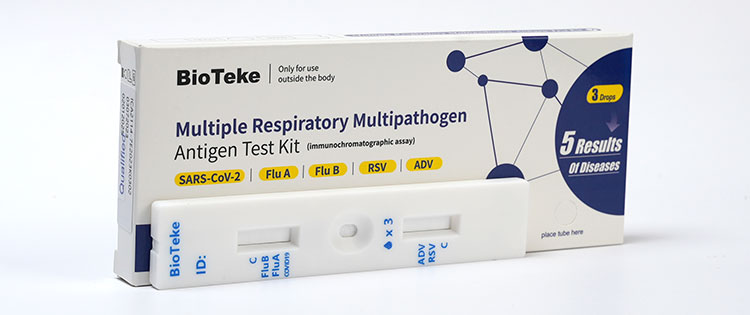
b. Multiple Respiratory Multipathogen Antigen Test Kit (5 Results of Diseases)
This kit can be detected five items: SARS-CoV-2、Influenza A virus 、Influenza B virus 、Respiratory syncytial virus and Adenovirus antigen.
c. Multiple Respiratory Multipathogen Antigen Test Kit (4 Results of Diseases)
This kit can be detected four items: SARS-CoV-2、Influenza A virus 、Influenza B virus and Respiratory syncytial virus.
(a)These antigen tests are included under “Category A” of the EU common list:
| Prospective clinical field study |
| Study in Poland.Sample size : 103 positive ,325 negative .Sensitivity:90.29% ; specificity:99.04% |

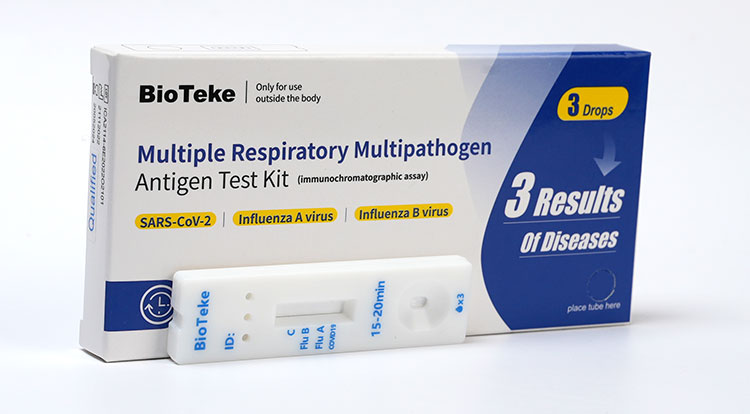
d. Multiple Respiratory Multipathogen Antigen Test Kit (3 Results of Diseases)
This kit can be detected three items: SARS-CoV-2、Influenza A virus and Influenza B.
E. Related Products
Multiple Respiratory Pathogens Nucleic Acid Detection Kit

E. Product qualifications(By Sept.22,2023)
| Multiple Respiratory multipathogen antigen test kit |
| Malaysia Registration |
| CE Certification – CIBG Registration Letter |
| CE Certification –CIBG Registration Prove |
| CE Certification–EC Declaration of Conformity |
| Export Qualified Suppliers |
| Free sale certificate-Malaysia |
| Free sale certificate-Mexico |
| Free sale certificate-Indonesia |
| Free sale certificate-Sri Lanka |
| Free sale certificate-United Arab Emirates |
| ISO 13485 Certificate |
| ISO 9001 Certificate |
| ISO 45001 Certificate |
| ISO 14001 Certificate |
| IPMS Certificate |
A. Intended Use:
When it comes to Covid 19, Flu A, Flu B ,Respiratory Syncytial Virus (RSV) ,Adenovirus(ADV) and Mycoplasma Pneumoniae(MP) testing, Results from Bioteke SARS-Cov-2/Flu A /Flu B /RSV/ADV /MP antigen test kit allow physicians to make a rapid diagnosis with confidence, which is essential for reducing secondary testing, decreasing unnecessary antibiotic use, and guiding appropriate antiviral therapy.
B. Advantages:
• Patent design - Two-way chromatography ,only single sample well.
• Compact card better friendly to the environment.
• Multi-color display results,Easy-to-read results in 15 minutes.
• High Sensitivity, Specificity and Accuracy, reliable results.
• Long shelf life to adapt to seasonal fluctuations.
• Variety of collection and transport options.
• Convenient room temperature storage.

C.Result Analysis
• SARS-CoV-2: Specificity: 100% ; Sensitivity 96.08% ; Total Coincidence Rate : 99.47%
• Influenza A : Specificity: 100% ; Sensitivity 98.4% ; Total Coincidence Rate : 99.82%
• Influenza B : Specificity: 100% ; Sensitivity 96.8% ; Total Coincidence Rate : 99.65%
• RSV : Specificity : 100% ; Sensitivity 97.98% ; Total Coincidence Rate : 99.82%
• Adenovirus : Specificity : 100% ;Sensitivity 95.83% ; Total Coincidence Rate : 99.56%
• M.Pneumoniae : Specificity: 100% ; Sensitivity 97.98% ; Total Coincidence Rate : 99.82%
D. Four Options:
a. SARS-CoV-2/Flu A/Flu B/RSV/ADV/MP Antigen Rapid Test Kit (6 Results of Diseases)
This kit can be detected six items: SARS-CoV-2 , Influenza A virus , Influenza B virus , Respiratory syncytial virus , Adenovirus antigen and Mycoplasma Pneumoniae.

b. Multiple Respiratory Multipathogen Antigen Test Kit (5 Results of Diseases)
This kit can be detected five items: SARS-CoV-2、Influenza A virus 、Influenza B virus 、Respiratory syncytial virus and Adenovirus antigen.

c. Multiple Respiratory Multipathogen Antigen Test Kit (4 Results of Diseases)
This kit can be detected four items: SARS-CoV-2、Influenza A virus 、Influenza B virus and Respiratory syncytial virus.
(a)These antigen tests are included under “Category A” of the EU common list:
| Prospective clinical field study |
| Study in Poland.Sample size : 103 positive ,325 negative .Sensitivity:90.29% ; specificity:99.04% |

d. Multiple Respiratory Multipathogen Antigen Test Kit (3 Results of Diseases)
This kit can be detected three items: SARS-CoV-2、Influenza A virus and Influenza B.

E. Related Products
Multiple Respiratory Pathogens Nucleic Acid Detection Kit

E. Product qualifications(By Sept.22,2023)
| Multiple Respiratory multipathogen antigen test kit |
| Malaysia Registration |
| CE Certification – CIBG Registration Letter |
| CE Certification –CIBG Registration Prove |
| CE Certification–EC Declaration of Conformity |
| Export Qualified Suppliers |
| Free sale certificate-Malaysia |
| Free sale certificate-Mexico |
| Free sale certificate-Indonesia |
| Free sale certificate-Sri Lanka |
| Free sale certificate-United Arab Emirates |
| ISO 13485 Certificate |
| ISO 9001 Certificate |
| ISO 45001 Certificate |
| ISO 14001 Certificate |
| IPMS Certificate |

Telephone/Whatsapp: +86 13357927939

Email : zr@bioteke.cn